
TREMOR
TREMOR: Safety and effectiveness of frameless linac-based stereotactic radiosurgery on tremor in patients with essential tremor or Parkinson’s disease
Trial summary:
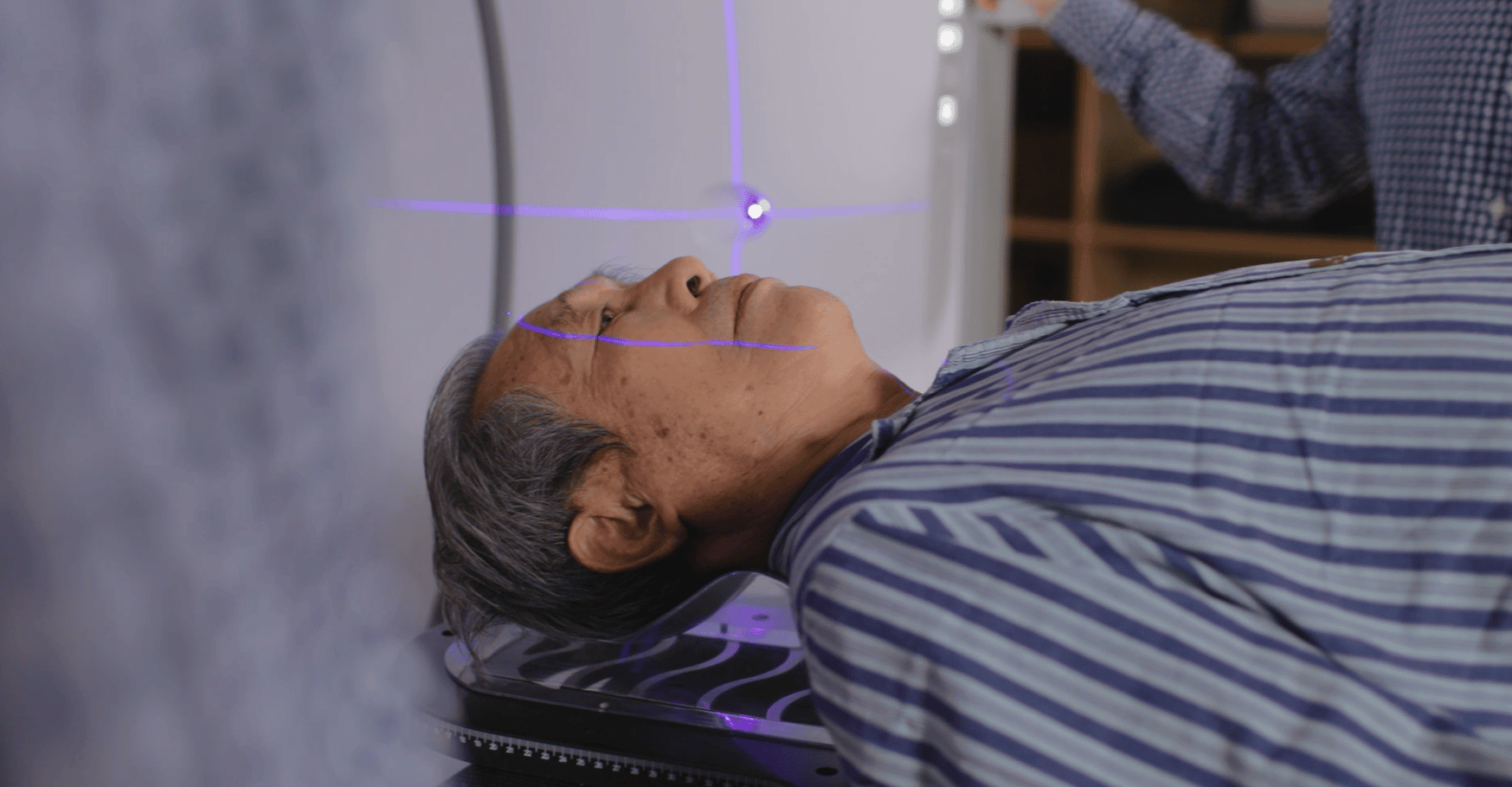
This study is a trial of frameless linac-based stereotactic radiosurgical thalamotomy using coordinate-based targeting of the ventral intermediate (VIM) nucleus. All participants will receive 130Gy to be delivered to Dmax utilising a 4mm tertiary conical collimator. This dose will be delivered in a single fraction (i.e. one treatment session) for most participants, but if participants are not able to complete the treatment in one session and have received less than 110Gy they will have the opportunity to receive the remaining radiation dose no later than the next day. The treatment will take approximately 1 hour.
Prior to treatment, participants will undergo a planning MRI and planning CT scan. Treatment will be planned and delivered at Icon Cancer Centre Richmond by a qualified investigator and radiation therapy team.
A number of quality assurance measures are in place to ensure all treatment plans are reviewed and approved by the appropriate parties (e.g. medical physicist) to ensure safety and accuracy. Post-treatment, imaging is taken and reviewed by radiation therapists to check the treatment delivery. All reviews will be documented in the medical record.
Receptor status / problem studied:
Inclusion criteria
1) Age great than or equal to 18 years.
2) Clinical diagnosis of essential tremor or tremor dominant Parkinson’s disease made by a neurologist.
3) Tremor of at least one upper limb resulting in significant disability despite medical treatment.
4) Medication refractory tremor or intolerant to medications.
5) Not a suitable surgical candidate or declined surgery.
6) Assessed as able to tolerate MRI scan and treatment session(s) with light sedation.
7) Has been reviewed and approved for enrolment by the multidisciplinary study team.
In addition, the following criteria will apply (to be assessed by the study movement disorders neurologist):
For patients with essential tremor:
|— a) tremor refractory to adequate trials of at least two medications, one of which should be a first line therapy of either propranolol or primidone (an adequate medication trial is defined as a therapeutic dose of each medication or the development of side effects as the medication dose is titrated) unless there are contraindications to them, or if assessed by a neurologist to have a severe tremor that would likely not have significant functional benefit from medications alone.
|— b) Postural and intention tremor severity score of greater than or equal to 2 in the extremity marked for treatment as measured by the FTMRS while stable on medication.
|— c) Significant disability due to essential tremor despite medical treatment (FTMRS score of 2 or above in any one of the items 16-21).
|— d) My have bilateral appendicular tremor.
For patients with tremor dominant Parkinson’s Disease:
|— a) will have a tremor dominant and postural instability/gait difficulty (TD/PIGD) ratio > 1.5 in the medicated [ON] state as calculated from the Unified Parkinson’s Disease Rating Scale (UPDRS) formula as described by Jankovic et al. (Jankovic et al. 1990)
|— b) tremor remains disabling when medical therapy is optimal or not tolerated for the treatment of other cardinal signs of Parkinson’s disease (bradykinesia, rigidity, etc.), as determined by a neurologist at the site
|— c) a resting tremor severity score of greater than or equal to 3 in the hand/arm as measured by the medicated (ON) UPDRS question #20 or a postural/action tremor greater than or equal to a 2 for question #21
|— d) subject exhibits a significant disability from their PD tremor despite medical treatment where a significant disability is defined as a Parkinson’s disease tremor with at least a score of 3 on #16 of the medicated (ON) UPDRS
Exclusion criteria
1) Presence of head tremor that would interfere with treatment delivery.
2) Patients with central neurodegenerative disease other than Parkinson’s disease.
3) Drug-induced parkinsonism.
4) Unable to undergo MRI due to the presence of a metallic implanted device such as a pacemaker or defibrillator or cochlear implant.
5) Prior cranial irradiation.
6) Change in medication(s) in the four weeks prior to enrolment or plans to change medication(s) during the 12-month follow-up period.
7) Presence of significant cognitive impairment as determined by meeting the DSM-5 criteria for Major Cognitive Disorder.
8) Unstable psychiatric disease, defined as active uncontrolled depressive symptoms, psychosis, delusions, hallucinations, or suicidal ideation. Subjects with stable, chronic anxiety or depressive disorders may be included provided their medications have been stable for at least 60 days prior to study entry and if deemed appropriately managed by the site neuropsychiatrist.
9) Unable to provide informed consent.
10) Unable or unwilling to attend follow-ups or comply with protocol requirements.
11) Pregnant or planning to become pregnant.
Trial Title
TREMOR
Diagnosis
Neurological conditions
Type of trial
Investigator Initiated
Type of treatement
Radiation Oncology
Phase
N/A